DNA repair and essential trace metals
The DNA, which contains all genetic information, can be damaged by exogenic and endogenic factors at every second. Exogenic factors include ionized radiation, ultraviolet (UV) radiation and a list of genotoxic substances. Even reactive oxygen species (ROS) like superoxide radical, hydroxyl radical, and hydrogen peroxide (H2O2) can induce DNA damage. Endogenic DNA damage are induced spontaneously, e.g. via desamination, oxidation of DNA bases and methylation. To secure genetic stability it is crucial to maintain an efficient repair system. Hereby, cells use different repair machanisms, depending on the induced damage.
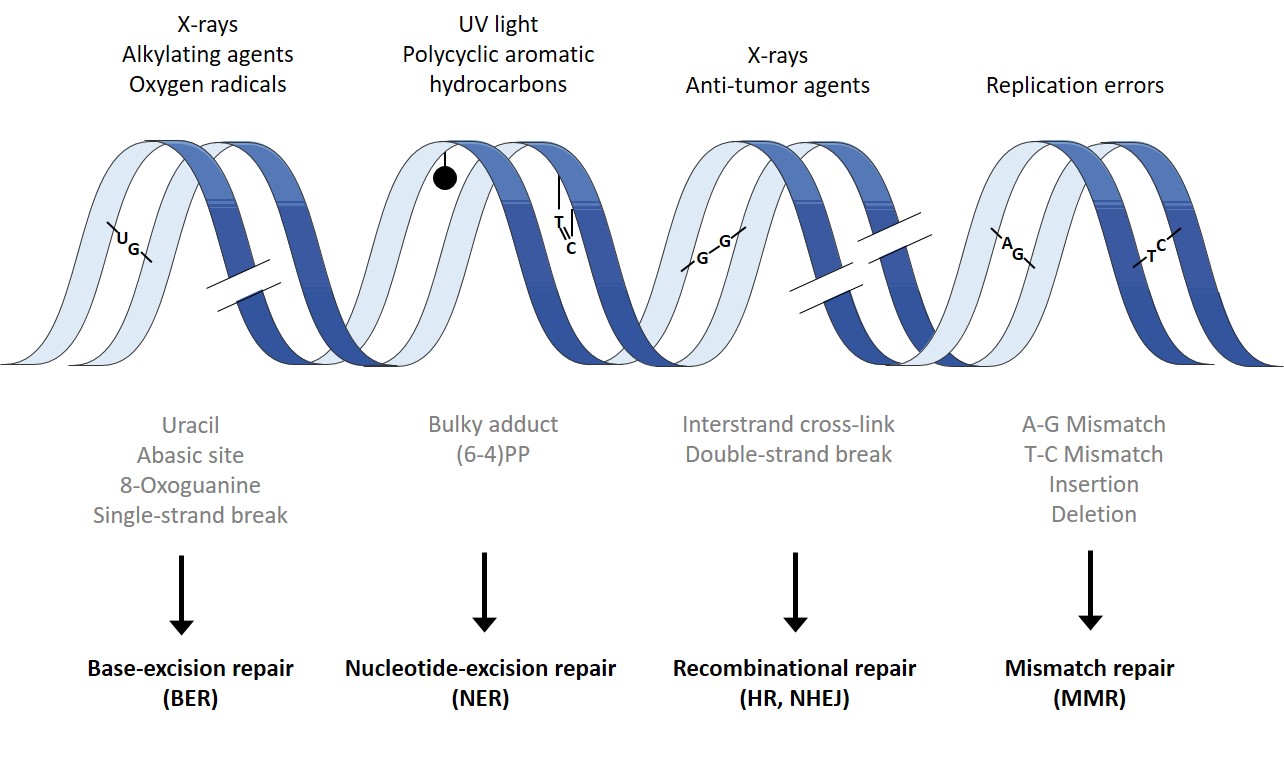
Small DNA lesions, such as the oxidation of DNA bases and DNA strand breaks, are repaired by base excision repair (BER) and single strand break repair (SSBR). Bulky adducts, such as pyrimidine dimers, are repaired via nucleotide excision repair (NER). DNA double strand breaks are repaired by two mechanisms: non homologous endjoining (NHEJ) and homologous recombination (HR). Lastly, mismatch repair (MMR) is present to correct errors during DNA replication.
Any disruption in one of these mechanisms results in unrepaired or insufficiently repaired damage, which in turn can lead to mutations, transcription and replication inhibition, apoptosis, necrosis, mutations, chromosomal aberrations, as well as loss of cell division control (cancer) and embryonic malformations (teratogenesis).
The effectiveness of existing repair mechanisms is therefore crucial for maintaining genomic stability. Among others, the DNA repair proteins PARP-1 (poly(ADP-ribose)-polymerase 1) or BRCA1 (Breast Cancer 1), which are research focuses in our working group, play a decisive role in this context. Both proteins have zinc-binding structures, which represent potential targets for toxic metal ions or (semi-)metal compounds such as those of inorganic arsenic and cadmium, and their function can thus be impaired. As an essential trace element, zinc is therefore significantly involved in maintaining genomic stability. However, at high concentrations, such as those possibly achieved by food supplements, toxic reactions for this metal cannot be excluded. The often close link between essential and toxic effects is particularly evident with trace elements such as copper and iron. While the essential biological function is to catalyse one-electron transitions, it is precisely this ability that can also lead to toxic reactions by catalysing the generation of reactive oxygen species that can subsequently damage cellular macromolecules. Thus, an exact regulation of metal ion concentrations in tissues and cells is necessary to prevent toxic effects; this is achieved by strict control of uptake and intracellular storage. Toxic effects occur when this homeostatic control is overridden either by excessive concentrations, by defects in uptake or storage, or by unphysiological uptake routes. In addition, the biochemical effect also depends decisively on the species in which the respective element is offered, for example as a food supplement. Three trace elements are studied in detail in our group: selenium, copper and zinc. In the case of selenium, we were able to show that the cellular effects depend decisively on the selenium species and especially on the oxidation state. While completely reduced selenium compounds are predominantly non-cytotoxic, reducible selenium compounds such as sodium selenite can, for example, oxidize zinc-complexing thiol groups and lead to inhibition of DNA repair processes and tumor suppressor functions. In the case of copper, inhibition of poly(ADP-ribosylation) and base excision repair occurs in the presence of cellular copper overload; in addition, copper ions as well as copper-containing nanoparticles lead to pronounced cellular stress reactions. Overall, the trace elements copper, selenium and zinc need to be balanced between sufficient uptake and avoidance of over-supply, thus preventing impairment of genomic stability.
